Introduction
Efficacy of SARS-CoV-2 vaccination is confirmed. In the real world, vaccination rates are low or vaccinated patients with hematologic malignancies are at increased risk of an inadequate response due to immunocompromised status. The PROVENT study demonstrated the safety and efficacy of the monoclonal antibody combination tixagevimab/cilgavimab (AZD7442/Evusheld). Pre-exposure prophylaxis is crucial for these patients to reduce the morbidity and mortality. However, there are limited data on hematological malignancies. Moreover, Korea government controls the vaccination schedule and tixagevimab/cilgavimab treatment uniformly. Notably, this trial was conducted during predominant omicron variant (BA.5) of SARS-CoV-2 in August 2022. In this study, we evaluated the efficacy and safety of tixagevimab/cilgavimab in patients with hematologic malignancies.
Method
Between 16 and 18 August 2022, 94 patients under active treatment for lymphoma, multiple myeloma, or acute leukemia at Ulsan University Hospital participated in this prospective observational study. All patients received a single dose AZD7442/Evusheld (two consecutive intramuscular injections of tixagevimab and cilgavimab, 300 mg each). Anti-SARS-CoV-2 spike protein antibody (Anti-S) titers and viral nucleocapsid antibody (Anti-N) were measured before administration of tixagevimab/cilgavimab and at 1 month, 3 months, and 6 months after administration.
Results
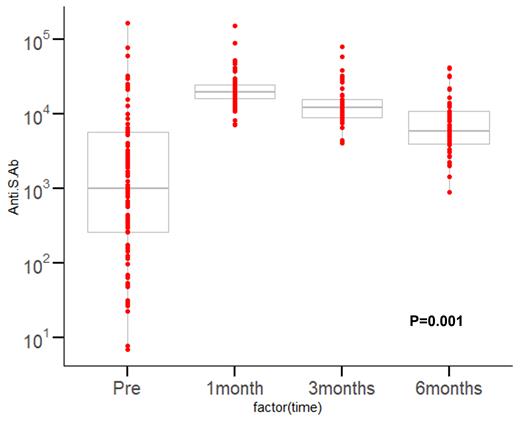
The median age was 64.5 years (37~ 85 years), and the male was 61.7%. The number of patients with ECOG 0-1 was 75 (79.8%). Forty-four patients were diagnosed with multiple myeloma (46.8%), 36 with lymphoma (38.3%) and 14 with acute leukemia (14.9%). A total of 28 patients (29.7%) underwent hematopoietic cell transplantation (HCT) (within 2 months for autologous HCT or 4 months for allogeneic HCT) or others received chemo-immunotherapy within 2 months from tixagevimab/cilgavimab administration. A total of 54 patients (57.4%) received first-line therapy prior to the administration of tixagevimab/cilgavimab. Twenty-five patients (26.6%) received Rituximab-based therapy. Twenty-five patients (26.6%) had previously confirmed SARS-Cov-2 infection. Fifty-nine patients (62.8%) had previously received SARS-Cov-2 vaccinations, with a median of two doses (0-5). A total of 29 patients (30.9%) had received vaccination prior to 6 months of tixagevimab/cilgavimab administration. Prior to administration, the median Anti-S titer was 862.8 AU/ml, and the Anti-N positive rate was 34%. On the other hand, the median was 20488.5 AU/mL at 1 month, 11983.0 AU/mL at 3 month, and 6479.0 AU/mL at 6 months after administration. As shown in the figure, all patients exhibited uniformly high levels of Anti-S after administration (p<0.001) and lasted for 6 months. A 14.9% rate of Anti-N positivity was observed six months after the administration of tixagevimab/cilgavimab. There was no significant safety concern with tixagevimab/cilgavimab. With a median follow-up time of 6 months after exposure of tixagevimab/cilgavimab, thirteen patients (13.8%) had documented SARS-Cov-2 infection. There was no SARS-Cov-2 related mortality.
Conclusions
Patients under active treatment due to hematologic malignancies are at high risk for severe SARS-Cov-2 infection because of age, comorbidity, humoral and cellular immune-compromises. The results of this study support the use of tixagevimab/cilgavimab for the prevention of symptomatic and severe SARS-Cov-2. SARS-Cov-2 prevention requires a layered strategy including vaccination, masking, and neutralizing antibodies that target the variant appropriately.
Figure. Anti-SARS-CoV-2 spike protein antibody (Anti-S) titers after admistration of tixagevimab/cilgavimab (AZD7442/Evusheld)
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal